Sugar Kelp Hatchery guidance - what went wrong?
-
Hi,
We have
successfully grown sugar kelp all the way from spores to maturity (10’ long
blades) but this year we failed 4 times to grow any seeds, and we are stumped
as to what the problem(s) are thus we’re reaching out to the community for
advice and guidance.
We followed the "Ocean Approved: Kelp Manual" as best we could. I've attached a word document with details and pictures of the process that we went through. I realize that this isn't a quick and dirty request but if anyone had time to read through this and offer suggestions or guidance I'd very much appreciate it.
Comments could be left here on the GW "community" or feel free to email or call me
Tks,
Mike
mike@pacificseafarms.com
206-491-0936
PSF Hatchery Process
Thanks for sharing this @mike_spranger. I converted your Word doc to a Google doc and have opened it up for comment access. That should help others to weigh in and collaborate to help troubleshoot. Find that doc here @toby_sheppardbloch @margaret_aydlett @david_bailey @lindsay_olsen Could you all take a look at Mike's description and comment as relevant? Please feel free to tag others who you think may have insights.
Thanks @Jamie_lee for adding comments to the doc as well!
@mike_spranger Hi Mike, thanks for sharing such a thorough write up! Here are some of my thoughts:
Overall, there are a lot of factors that could have caused the issues. To narrow it down, I think you can start by assuming that everything that worked well for your good nursery season was not the issue this season (ie. water temp, using instant ocean, pH, salinity, stocking density, nutrient dosing, etc). If I’m understanding everything correctly, that leaves the water conditioner as the only variable, but I think it is unlikely that the conditioner caused the issues. I see sporophytes in some of the photos you shared, so it does not seem like the season was a complete bust, but the sporophytes were definitely smothered by contaminants. I would suggest using a filter for the spore solution and doing more frequent water changes in the upcoming season to help keeps contamination down.
Did you outplant any of the seed even though you weren’t happy with it? The photos of your lines look great!
@margaret_aydlett Holy Cow, Margaret. Thank you for such a thorough response. I did make an error on the spore count I added per spool. I multiplied the amount of spores by the volume of my holding tubes to get the right volume of solution per holding tube/spool such that I was at 7500 spores
I’m glad that that dechlorination solution wasn’t a seaweed killer but I kind of wish it was so that I could ID that as my issue.
I need to get better educated on identifying what I’m looking at under the microscope plus I will pay more attention to the cleanliness of the water (change weekly) and your other suggestions
Do you have thoughts on the quality of the spores as the season progresses? In other words, my first attempt was with sorus collected in late November whereas the 2nd attempt was collected in January In both cases, to me the sorus was good and unfouled and the spores were highly mobile. As the season goes on do the spores “get tired” or otherwise degrade such that the likelihood of them producing seed goes down?
finally, I was going to out plant the seed string on my lines anyway but the wild set was so thick that I didn’t want to disturb or otherwise damage that so my crop this year will be entirely wild set
@margaret_aydlett hi Maggie, do you have any thoughts on this item? It may have gotten lost in all the details…
Do you have thoughts on the quality of the spores as the season progresses? In other words, my first attempt was with sorus collected in late November whereas the 2nd attempt was collected in January In both cases, to me the sorus was good and unfouled and the spores were highly mobile. As the season goes on do the spores “get tired” or otherwise degrade such that the likelihood of them producing seed goes down?
@mike_spranger Hi Mike, our rule of thumb is that motile spores are motile spores. In southern New England, we certainly see sorus collected later in the season having more biofouling on it, but otherwise haven't noticed many differences.
@margaret_aydlett Holy Cow, Margaret. Thank you for such a thorough response. I did make an error on the spore count I added per spool. I multiplied the amount of spores by the volume of my holding tubes to get the right volume of solution per holding tube/spool such that I was at 7500 spores
I’m glad that that dechlorination solution wasn’t a seaweed killer but I kind of wish it was so that I could ID that as my issue.
I need to get better educated on identifying what I’m looking at under the microscope plus I will pay more attention to the cleanliness of the water (change weekly) and your other suggestions
Do you have thoughts on the quality of the spores as the season progresses? In other words, my first attempt was with sorus collected in late November whereas the 2nd attempt was collected in January In both cases, to me the sorus was good and unfouled and the spores were highly mobile. As the season goes on do the spores “get tired” or otherwise degrade such that the likelihood of them producing seed goes down?
finally, I was going to out plant the seed string on my lines anyway but the wild set was so thick that I didn’t want to disturb or otherwise damage that so my crop this year will be entirely wild set
Thanks @Jamie_lee for adding comments to the doc as well!
@margaret_aydlett hi Maggie, do you have any thoughts on this item? It may have gotten lost in all the details…
Do you have thoughts on the quality of the spores as the season progresses? In other words, my first attempt was with sorus collected in late November whereas the 2nd attempt was collected in January In both cases, to me the sorus was good and unfouled and the spores were highly mobile. As the season goes on do the spores “get tired” or otherwise degrade such that the likelihood of them producing seed goes down?
@mike_spranger Hi Mike, our rule of thumb is that motile spores are motile spores. In southern New England, we certainly see sorus collected later in the season having more biofouling on it, but otherwise haven't noticed many differences.
@mike_spranger Hi Mike! I know diatoms can be a real threat to growing spores. I recommend to try to remove the silicate from the water that you are using. There are various methods that you can find online (E.g. filtering, RO, Calciumhydroxide). Because the diatoms are using the silicate to grow and reproduce, taking it away should significantly reduce the potential growth. Germanium dioxide would be a nice addition, but you mentioned it's very difficult to obtain for you.
My recommendations:
All the best,
Tristan
@tristan_s hi Tristan. I’m using Instant Ocean. Can you advise if this has silicates that should be avoided?
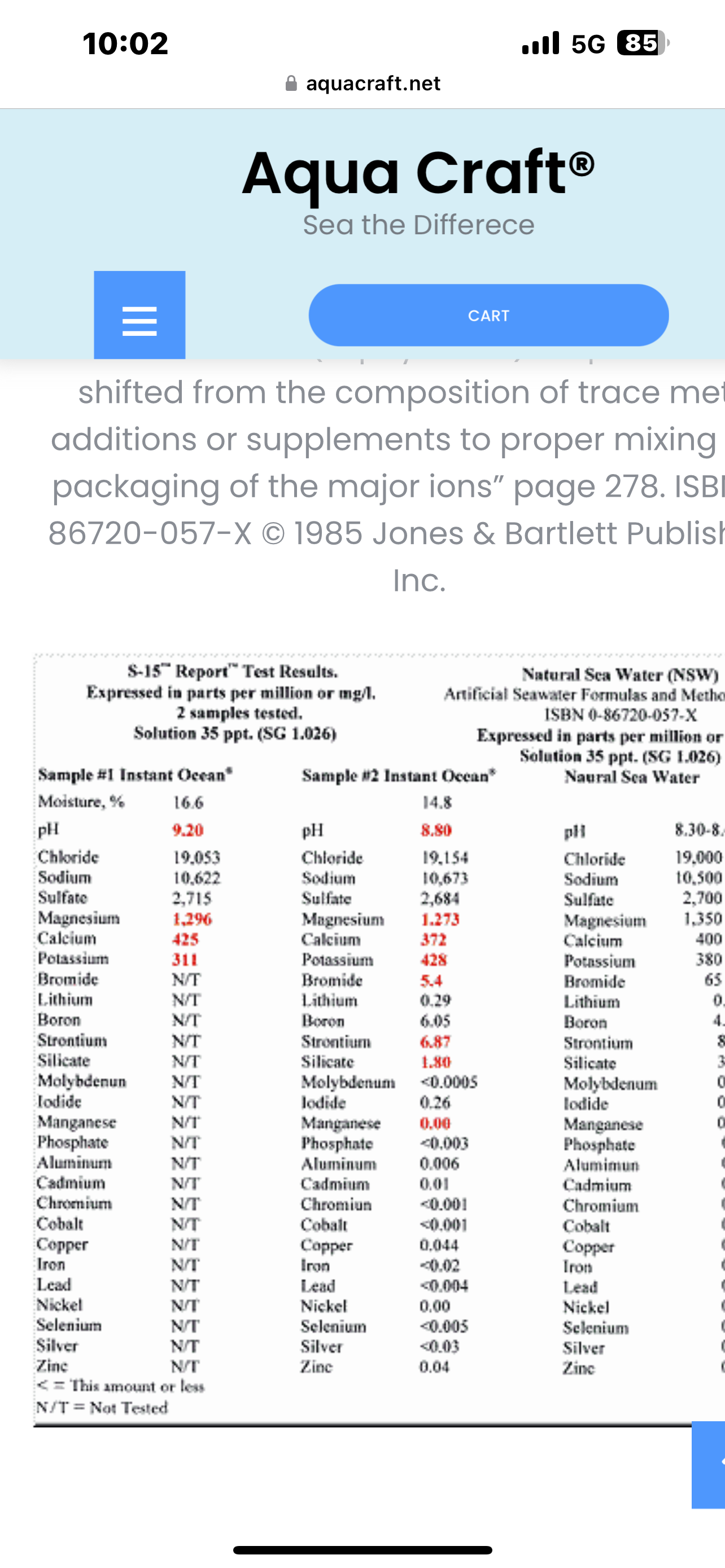
@tristan_s hi Tristan. I’m using Instant Ocean. Can you advise if this has silicates that should be avoided?

@mike_spranger The report that you provided states that the second sample has 1.8 mg/l of silicate in it. The recommended amount should be below 1.0 mg/l to combat diatom growth. It's interesting to see that there are almost no trace minerals in the first sample. Just out of curiosity, what is the difference between the two samples?
@tristan_s hey Tristan, I may have posted a chart that confused matters… I googled instant ocean ingredients/specifications and that’s what I got. I’m not setup to use actual ocean water and I believe instant ocean is the best and most widely used alternative for this purpose so I’m not sure what other choices I have…
@mike_spranger Hey mike, I think all the marine salt may contain traces of silicate. If would recommend treating the the marine salt before using it for hatchery purposes.
I would do the following:
The caustic soda will form white flakes that you can fish out with a fine mesh simply pass the solution over a cloth.
The caustic soda treatment can influence the pH (>9) so double check the pH once you have the final volume.
I hope this helps! Cheers!
@tristan_s hey Tristan, I may have posted a chart that confused matters… I googled instant ocean ingredients/specifications and that’s what I got. I’m not setup to use actual ocean water and I believe instant ocean is the best and most widely used alternative for this purpose so I’m not sure what other choices I have…
@mike_spranger Hey mike, I think all the marine salt may contain traces of silicate. If would recommend treating the the marine salt before using it for hatchery purposes.
I would do the following:
The caustic soda will form white flakes that you can fish out with a fine mesh simply pass the solution over a cloth.
The caustic soda treatment can influence the pH (>9) so double check the pH once you have the final volume.
I hope this helps! Cheers!